
The PRISMA consort flow diagram showing the process of study selection | Download Scientific Diagram

PDF) Use of reporting guidelines in scientific writing: PRISMA, CONSORT, STROBE, STARD and other resources

Reporting guidelines of health research studies are frequently used inappropriately - Journal of Clinical Epidemiology

PRISMA CONSORT diagram showing identification and screening of studies... | Download Scientific Diagram

Knowledge translation concerns for the CONSORT-PRO extension reporting guidance: a review of reviews | SpringerLink

Study flow chart. CONSORT, CONsolidated Standards of Reporting Trials;... | Download Scientific Diagram
An Investigation of the Shortcomings of the CONSORT 2010 Statement for the Reporting of Group Sequential Randomised Controlled Trials: A Methodological Systematic Review | PLOS ONE

PRISMA consort diagram of how the literature was screened. *Trivial... | Download Scientific Diagram
Use of Reporting Guidelines in Scientific Writing: PRISMA, CONSORT, STROBE, STARD and Other Resources

The PRISMA 2020 statement: An updated guideline for reporting systematic reviews - Journal of Clinical Epidemiology
![The relationship between endorsing reporting guidelines or trial registration and the impact factor or total citations in surgical journals [PeerJ] The relationship between endorsing reporting guidelines or trial registration and the impact factor or total citations in surgical journals [PeerJ]](https://dfzljdn9uc3pi.cloudfront.net/2022/12837/1/fig-2-2x.jpg)
The relationship between endorsing reporting guidelines or trial registration and the impact factor or total citations in surgical journals [PeerJ]

Literature search PRISMA consort diagram PRISMA diagram of systematic... | Download Scientific Diagram

SpinalCord on Twitter: "To enhance the quality and transparency of health research it is recommended to follow the reporting guidelines for the different study types. See the @EQUATORNetwork website for all guidelines:
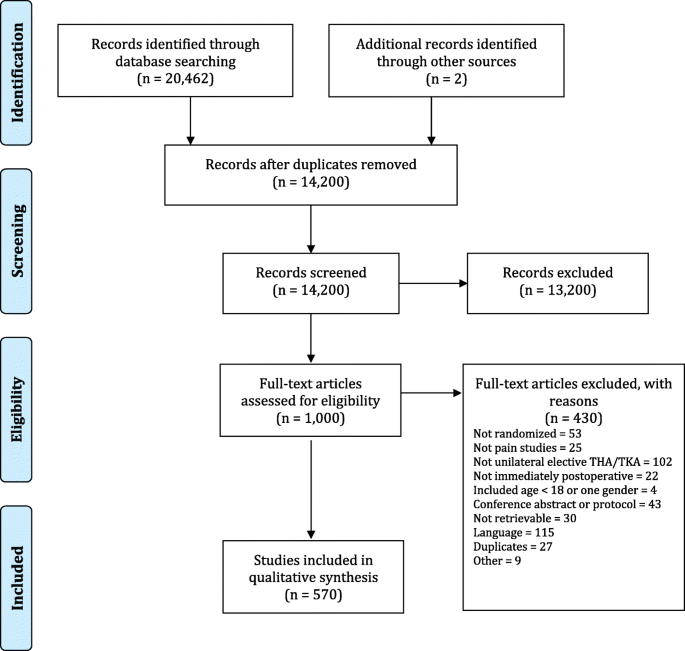
Adherence to participant flow diagrams in trials on postoperative pain management after total hip and knee arthroplasty: a methodological review | Trials | Full Text

Relation of completeness of reporting of health research to journals' endorsement of reporting guidelines: systematic review | The BMJ

CONSORT and QUOROM guidelines for reporting randomized clinical trials and systematic reviews - American Journal of Orthodontics and Dentofacial Orthopedics









![PDF] Reporting guidelines for implementation and operational research | Semantic Scholar PDF] Reporting guidelines for implementation and operational research | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e8b893b2a4875df7365787913e81b8afb74a8619/5-Table3-1.png)